This is the 3rd part of principles of entrainment – please see entrainment -1 and entrainment -2 before you read this if not already done.
Before going further, we need to understand the concept of fusion.
As per the literal meaning this is about some thing blending together. In EP, this is mostly applied to surface ECGs -but intra-cardiac electrograms also can have fusion.
So what is fusion in ECGs? It is basically the combined effect of two or more foci of depolarization on the ECG. Lets look at very obvious example – Pre-excited ECG of WPW. In this, when a sinus beat comes down, it will go thru the conduction system as well as the accessory pathway. The contribution by the AP will give rise to the delta wave and the rest of the QRS will be by the conduction system. In the end, we have a fused complex of the delta wave with the QRS complex. If we remove the accessory pathway conduction – we have a pure QRS complex – the message here is that to identify fusion, a difference in the ECG morphology must be seen – therefore for practical purposes, most attempts at identifying fusion is applied to QRS complexes -not P waves as identifying a fused (different) p wave can be challenging. Read it carefully : if you cannot see a difference in ECG morphology, then there is no manifest (apparent) fusion !
In entrainment this refers to the combined (fused) appearance of ECGs during pacing.
Let’s work it out by using the following diagram. This is a bit different from the previous cartoons that we have seen and is closer to the structure of an actual tachycardia circuit – i.e. a scar VT circuit. But the same principles apply – there is a re-entrant circuit and there is an excitable gap we would like to penetrate. The major difference is that we now we consider the origin of the surface ECG and also look for fusion.
The tachycardia circuit consists of a slowly conducing narrow channel confined by two zones of scarring. This sets up a re-entrant circuit (see re-entry) . The ECG during tachycardia is generated by the orthordomic wavefront exiting at one end of the channel.

Now assume that we place a catheter at the very exit site and pace slightly faster than the tachycardia cycle length as shown :

The same thing would happen if we pace within the channel. Since the surrounding myocardium cannot be capture (i.e. scar), the surface ECG will be dependent on the exiting tachycardia at the exit. Again in technical jargon, no manifest fusion of the surface ECG!

Lack of any change in the tachycardia morphology during pacing (i.e. No manifest fusion of the ECG) – but fulflling other criteria for entrainment is called concealed entrainment
Now lets see what will happen if we pace on the outer myocardium (as would be a common practical scenario when we are looking for the circuit – aka pacing at random places with a mapping catheter).


As explained in the above diagrams, ECG fusion (a difference observed) is expected when pacing from an outer site. Once the paced wave penetrates the excitable gap, there would be continuous resetting of the tachycardia (antidromic colliding with the previous active front and orthodromic continuing as the tachycardia beat).
The main observation here is that ECG fusion is dependent on the site of pacing – therefore the absence of fusion in the surface ECG does not exclude re-entry. (in the next section entrainment – 4 we will see some practical examples – a very common entrainment that we do – pacing entrainment from the ventricle for AVNRT and AVRT do not show fusion at the surface ECG !!!)
Now what will happen if we pace at different faster rates? Look at the following diagram –

Sometimes ECG demonstration of fusion may not be possible. For example, in atria, say during analysis of a atrial tachycardia, demonstration of fusion in P waves is very difficult.
However if we have additional electrodes in the circuit (or in the vicinity) we can demonstrate fusion at an electrogram level. This is shown in the following diagram. Basically with faster pacing, the collision site is pushed downstream and a strategically placed electrode will show this movement with changes in morphology (direction) and distance to paced spike

As demonstrated in the above diagrams, progressive fusion is an important criterion to prove re-entry. Unfortunately not all pacing attempts demonstrate ECG fusion as fusion is dependent on the site of pacing.However one can try to look for evidence of electrogram level fusion to identify re-entry.
To conclude the discussion on ECG fusion, lets look at what happens when we abruptly stop pacing –
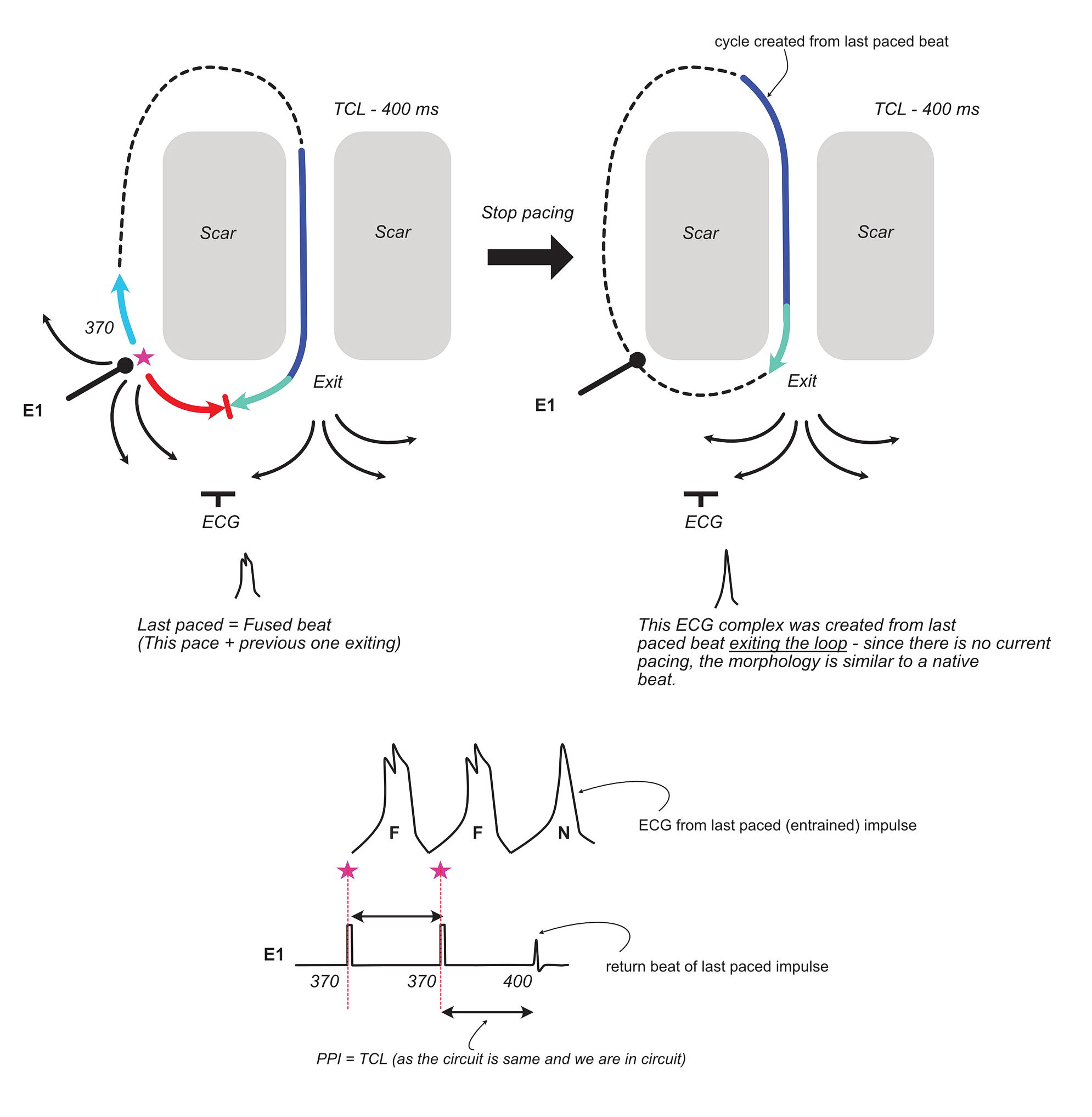
In addition to the fact that the ECG of the last cycle initiated by the paced beat appears like the native beat (no fusion), the other important takeaway is that the last beat cycle length is equal to the tachycardia cycle length (if we are in the circuit). In the original description of this phenomenon, it was stated that the last beat (also called the entrained beat) is at the paced cycle length – but it is obvious from the above that it has to be at the native cycle length and this has been proven in studies on human VTs
Now to the last bit – before we consolidate what we have learnt so far.
What will happen if we pace very fast than the tachycardia cycle length – i.e. make our paced beat enter the excitable gap very early?
Lets work this out with the following diagram :
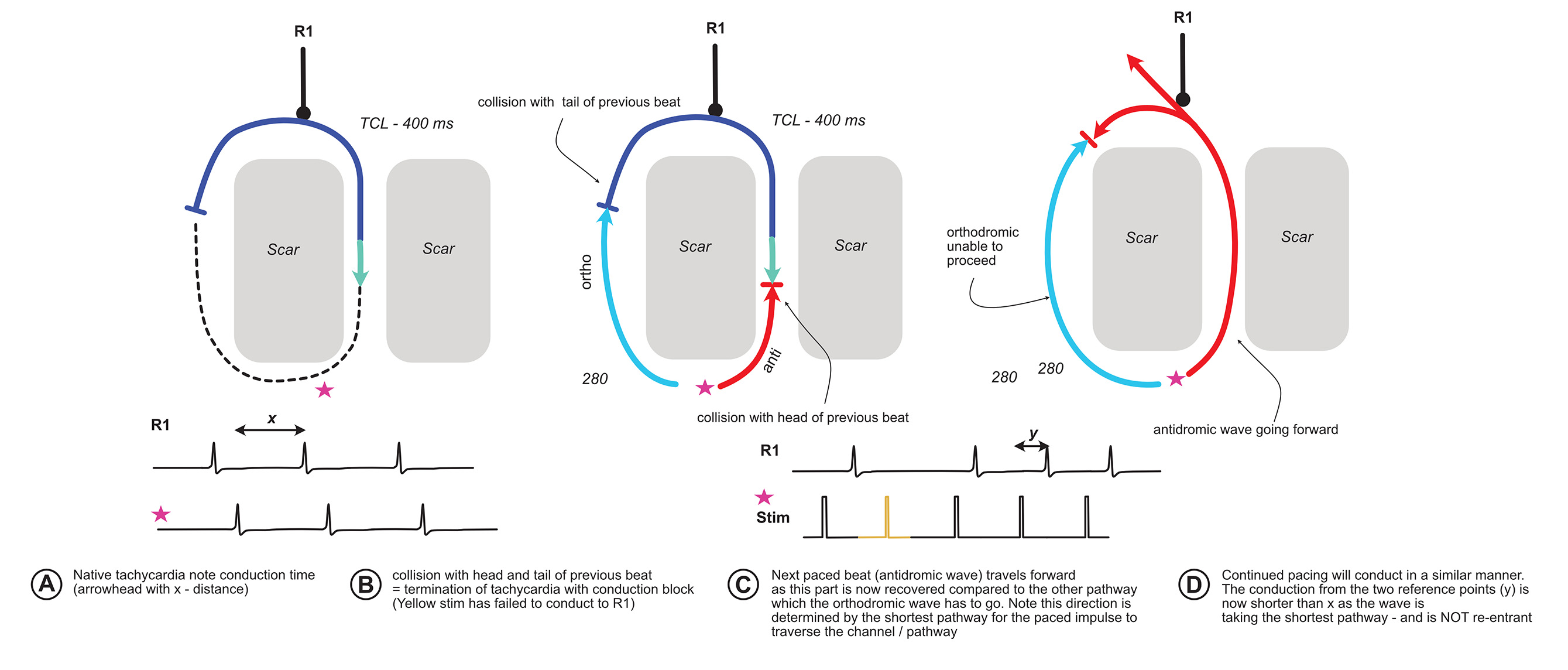
The critical observation of the above is that when there is termination of tachycardia with a conduction block, the subsequent paced beats activate the circuit in a different direction. For this phenomenon to occur, initial tachycardia has to be re-entry.
A relevant real life example is pacing the ventricle in orthodromic AVRT. Once a critical pacing speed is achieved, the tachycardia terminates with a block to the atrium and ventricle. The subsequent paced beat enters the atrium via the AV node limb (because our site of pacing is near the RV apex). If we have sufficient catheters, we will be able to see the different activation sequence (VAH before block vs VHA after block) AND the timing difference shorter.
Now lets recap what we learnt above in the form of official criteria for entrainment. Waldo originally described 3 criteria and the fourth one was added later by Henthorn. I deliberately avoided stating these criteria at the beginning to facilitate understanding the principles behind these criteria.
- Pacing during tachycardia yields constant fusion on the surface ECG except for the last captured beat, which is not fused
- Progressive fusion while pacing at different rates
- Pacing termination of tachycardia yields localized conduction block followed by activation of that site with a shorter conduction time
- Change in conduction time and electrogram morphology when pacing at 2 different rates (progressive fusion at electrogram level)
Now that you know the mechanisms, the criteria are self explantatory. Proving one or more criteria in a given tachycardia confirms its mechanism as re-entry.
With regard to the first criterion, identification of proper fusion is essential – sometimes this is not straightforward. If there is no change in surface ECG morophlogy – but yet the tachycardia fullfills other criteria, then we have concealed entrainment (i.e. entrainment without apparent ECG fusion). Concealed entrainment should be strictly applied to surface ECG as even in the absence of ECG fusion, 4th criterion may be fulfilled.
The conduction time clause (short) in the 3rd criterion is based on the fact that the paced beat travels via the antidromic route of the original circuit. However in real life other structures involved may add decremental properties to slow down and actually prolong the interval (the classic example being AV node dependent circuits) – the aim is to identify the different route compared to the native tachycardia
In the next section we will look at some practical examples of the above mechanisms at work