As the name implies, re-entry is something entering something in a repeated fashion. In arrhythmia, it is when a tachycardia is seen traveling (propagating) along an anatomical pathway.
Understanding what re-entry is and how re-entry tachycardia is formed is fundamental to understanding entrainment.Therefore this section will concentrate in what re-entry is and how re-entry is initiated.
Re-entry is one mechanism of arrhythmia and is the most fun type of mechanism. Other types include – triggered activity and automaticity (boring!) – why is reentry fun ? Because we can do things to it and most are potentially curable!
If you recall basic cellular electrophysiology, cardiac cells propagate impulses by depolarization not by direct conduction as in a electric circuit.
Depolarization and repolarization takes time (milliseconds). Therefore a spread of depolarization is followed by a tail of cells repolarizing. These repolarizing cells cannot excite until fully repolarized therefore they create a tail of refractory cells. The fully recovered cells in behind the refractory tail and the still not-depolarized cells in front of the wavefront are excitable and termed the ‘excitable‘ gap. The direction of the tachycardia depends on where the initial depolarization began and the anatomical tissue pathway.

Cardiac cells spread the depolarization to adjacent cells by gap junctions and this determines the microscopic direction of impulse conduction – however for practical purposes, macrosopcically, the impulse can travel in any direction as there are gap junctions all over the place. In some places (e.g. the Crista Terminalis) fiber orientation tends to direct the wave front.
For re-entry to occur, the depolarizing wavefront needs to come back on its own. For this to occur – there must be an anatomic obstruction that creates “circuit of tissue”. In real life these obstructions are either scar tissue or natural obstructions like valve rings, cavo-tricuspid isthmus etc.
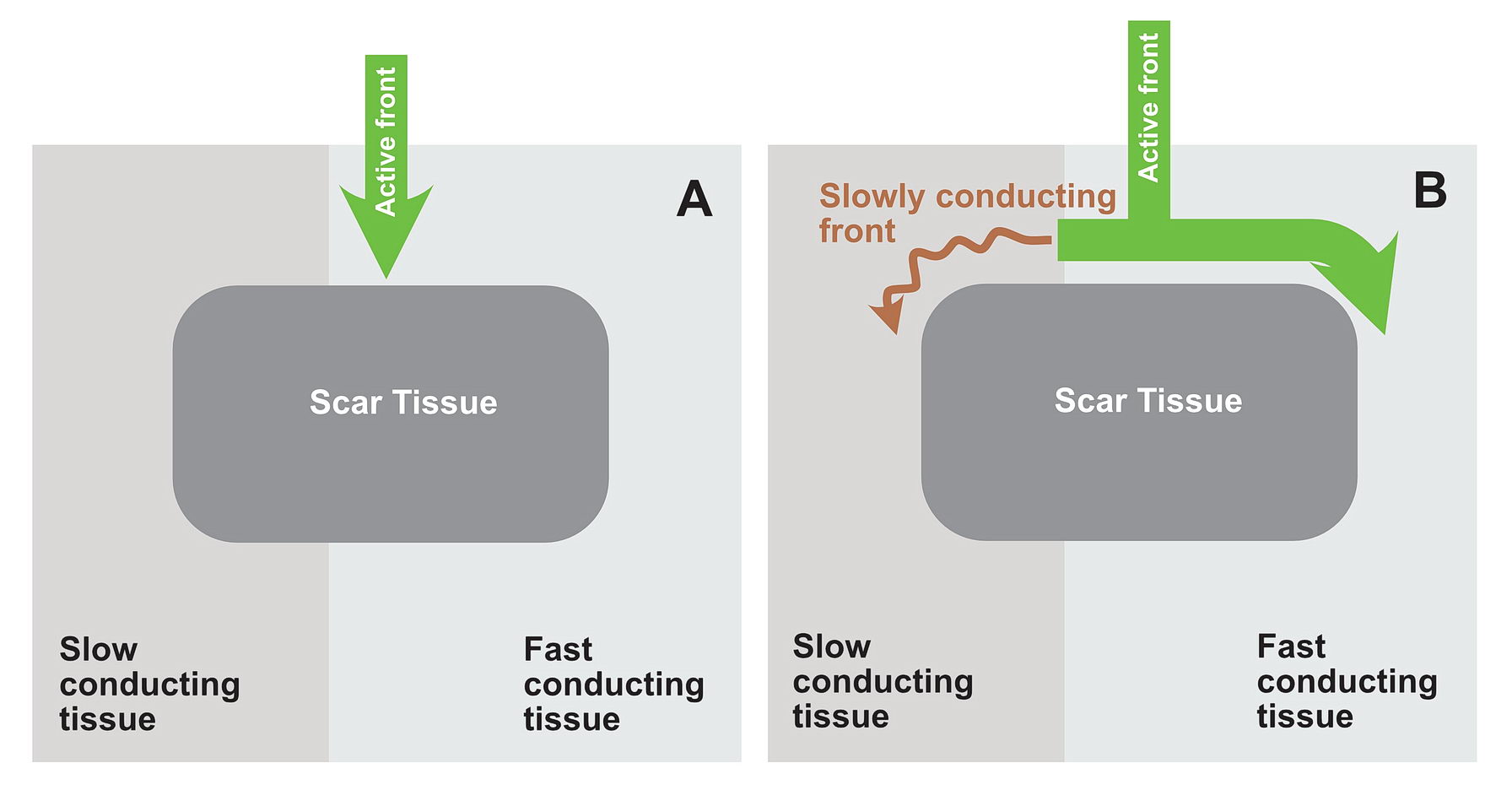
Lets look at the following diagram – which shows a anatomical scar within a blob of healthy tissue. What will happen if an impulse arrives at near the obstruction? Is re-entry possible?
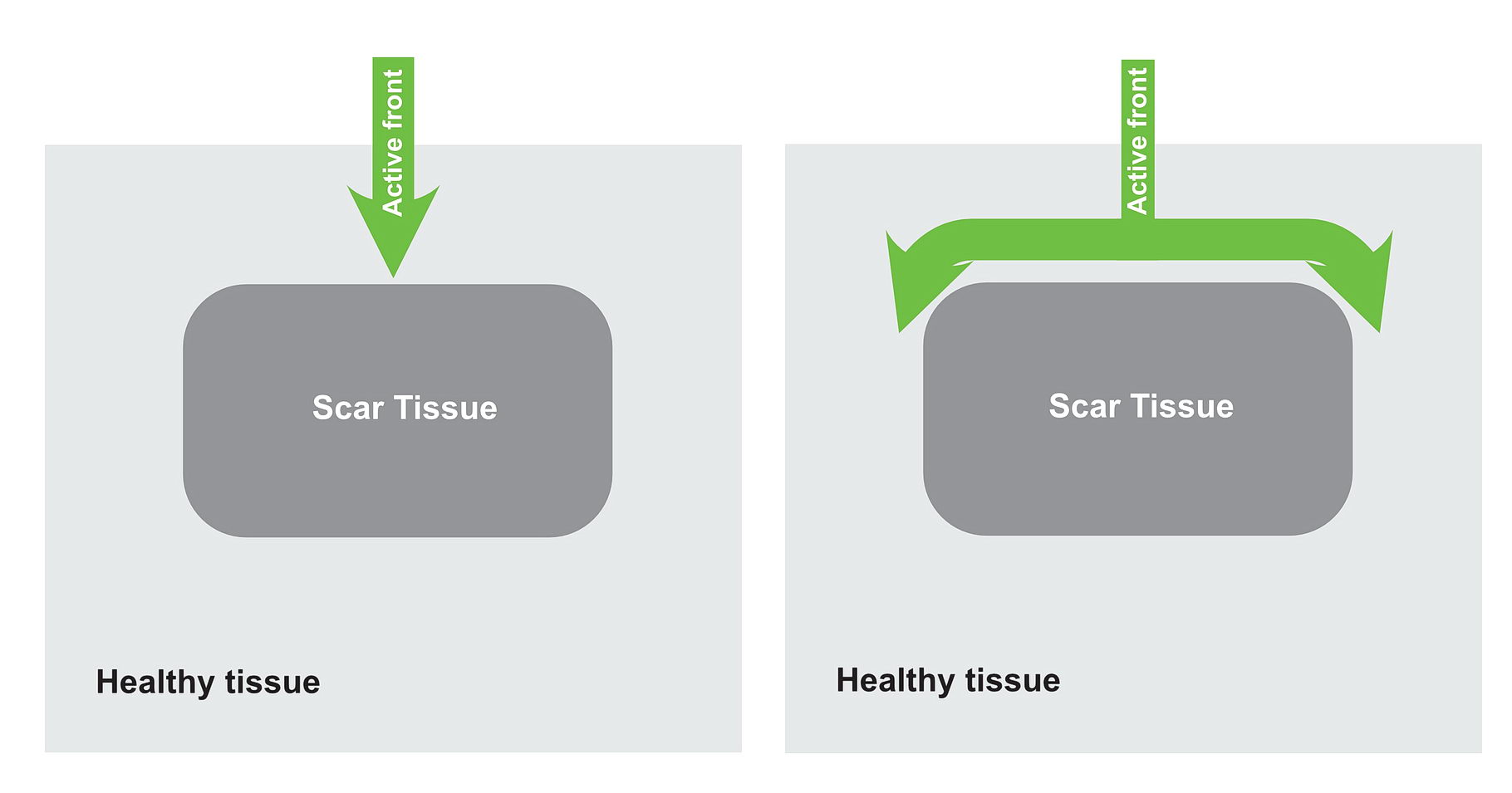
Now consider, the obstruction creating two pathways – which have different electrical properties. One can conduct fast and the other conducts slowly because of disease state.
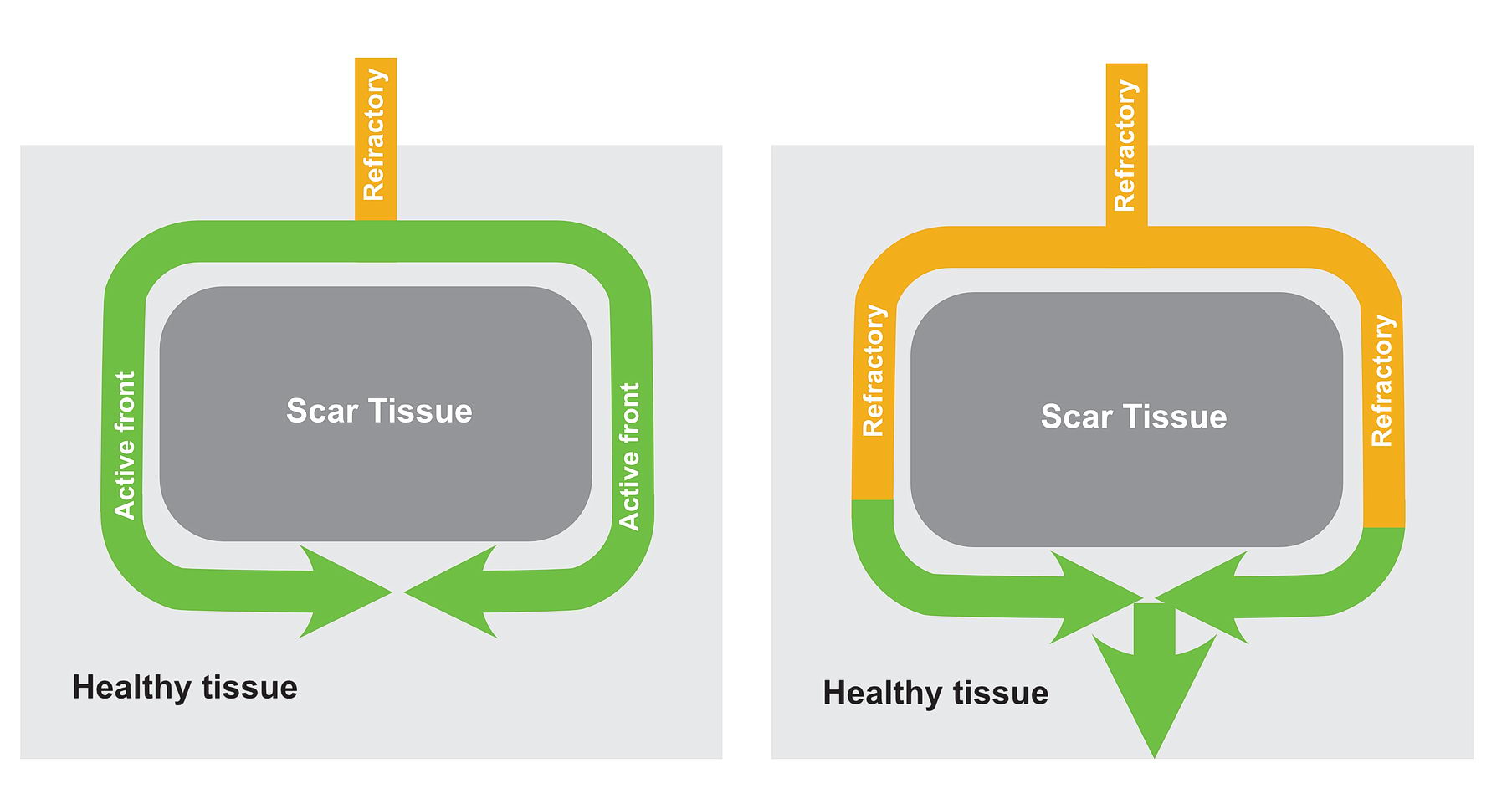
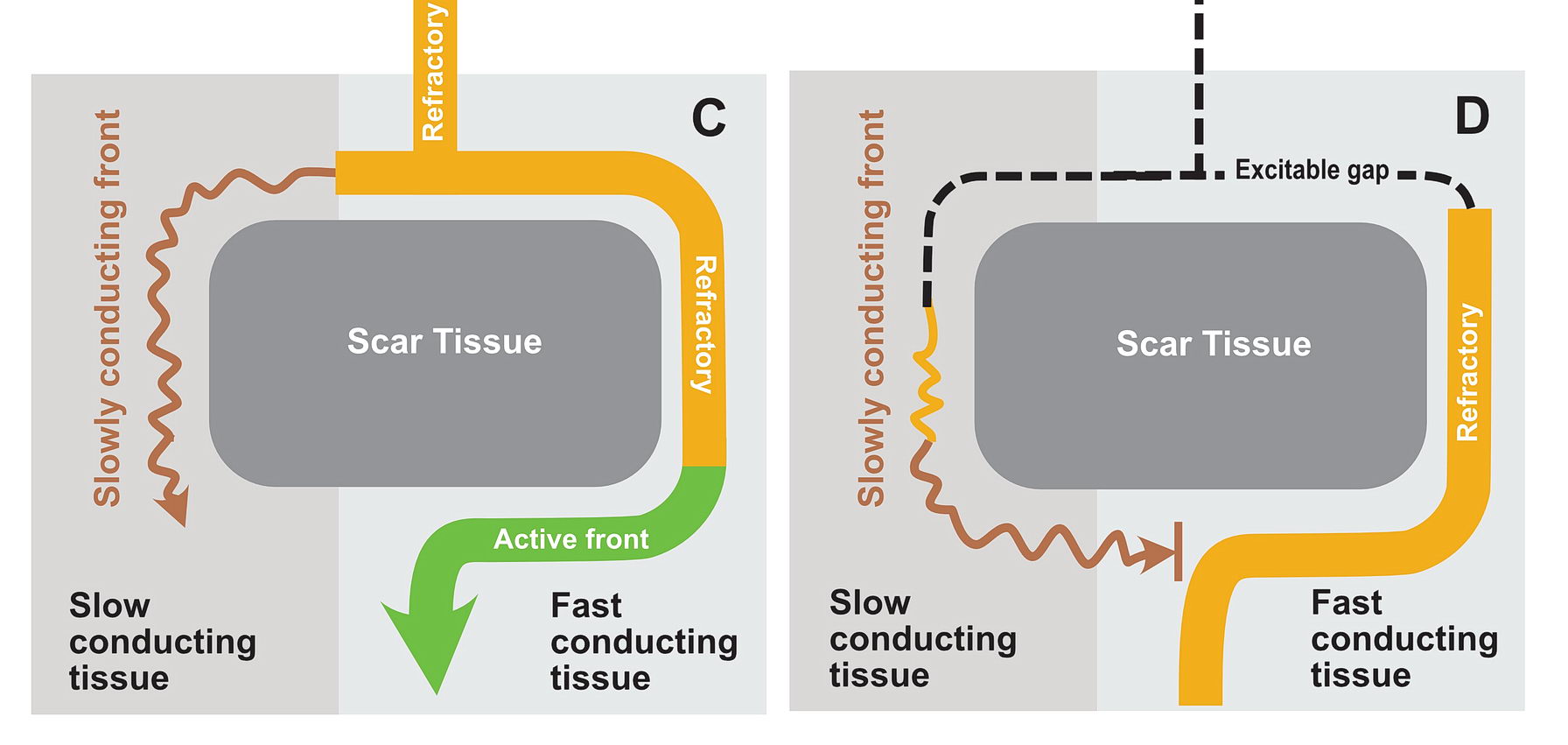
In this situation as shown in the diagrams, there exists a potential for the wavefront coming via the slow pathway to re-enter the other pathway provided the tissue is not refractory.
If the conduction via the slow pathway is sufficiently slow, the other pathway might have enough time to recover and enable re-entry
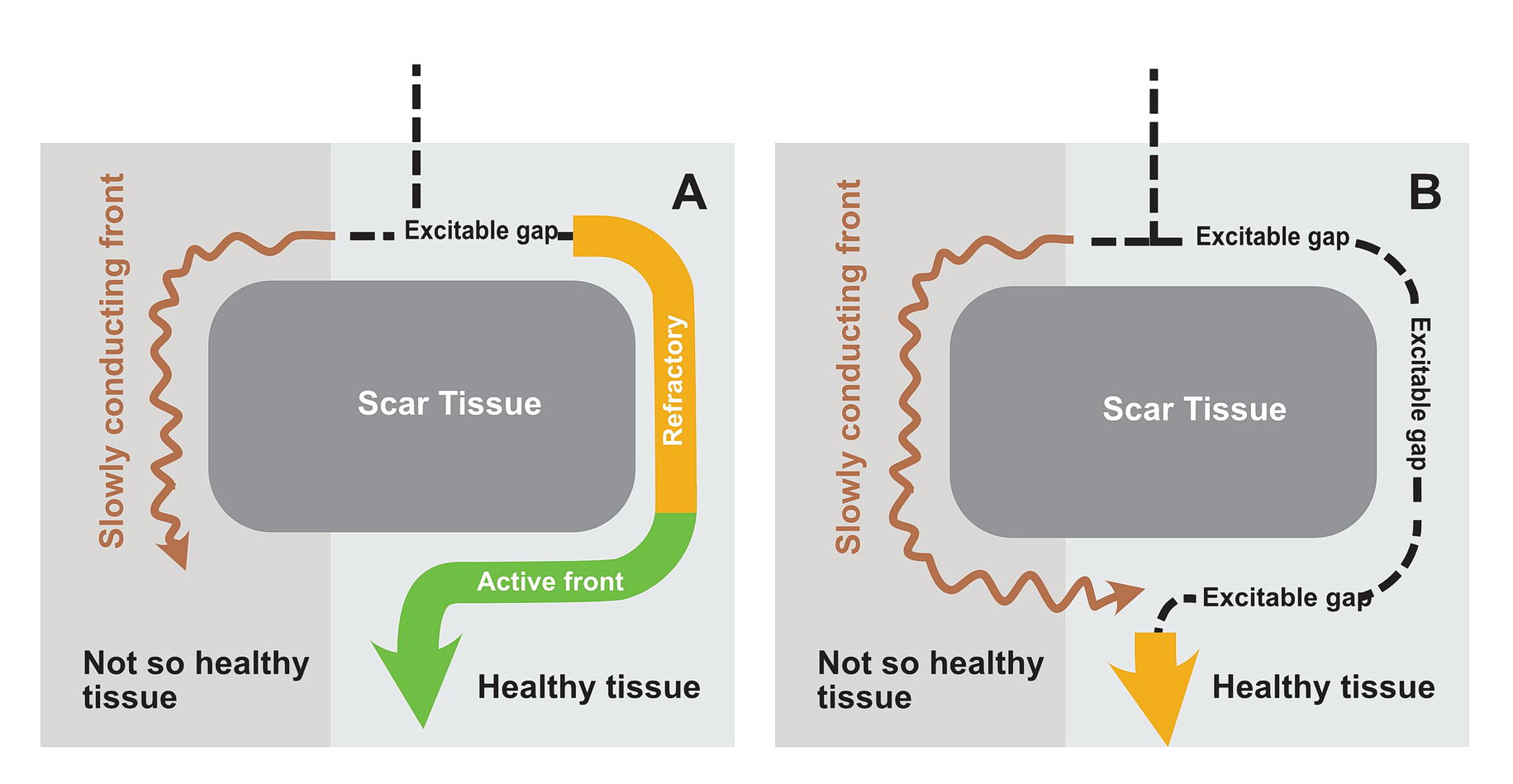
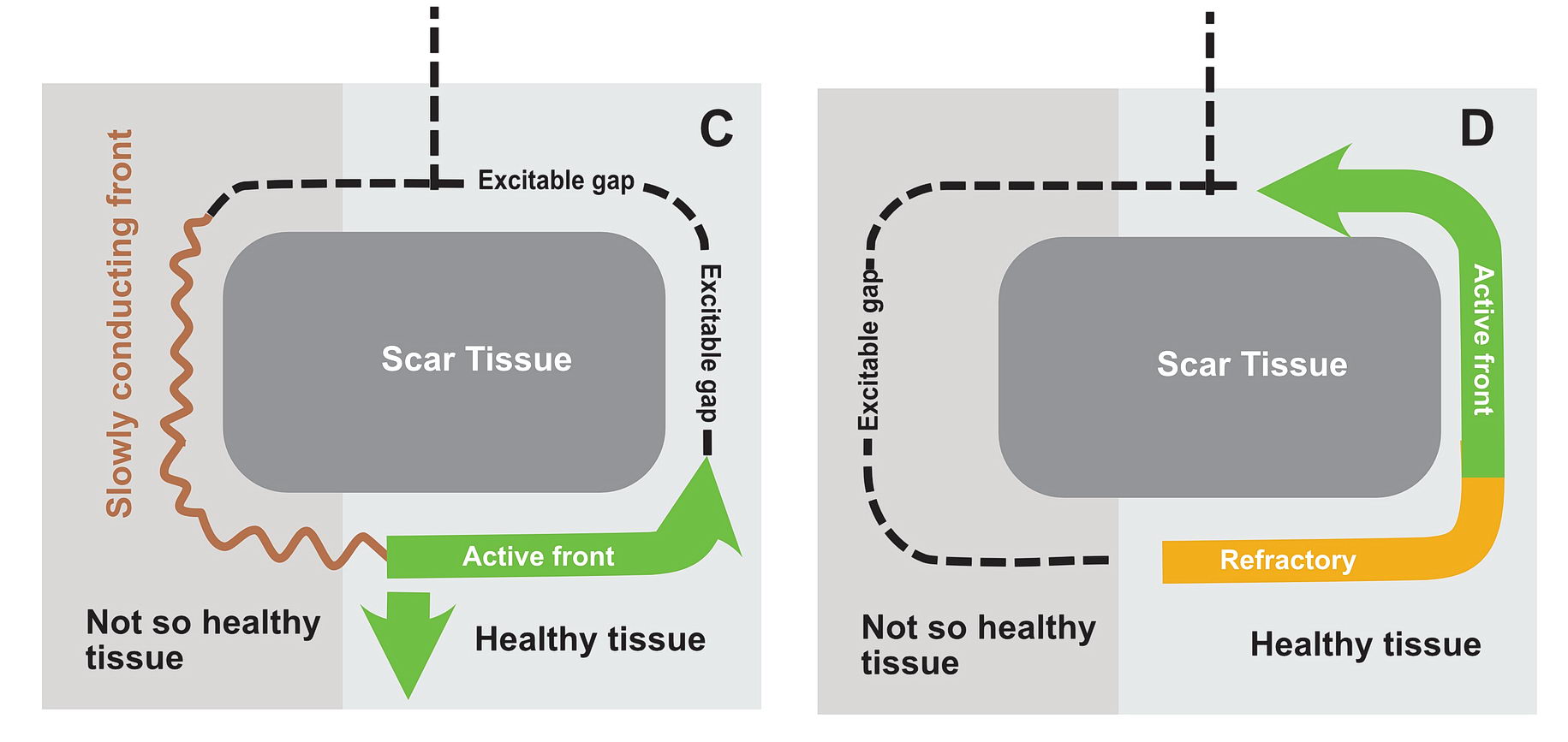
In real life this would mean incessant tachycardia and fortunately it is rare. The common mode of initiation of tachycardia when this sort of substrate is present by creating a functional unidirectional block and presence of premature impulses.
Lets look at this in detail as this is a very important concept for arrhythmia initiation which we can also use in the EP lab.
The requirements are
- Two sets of tissues – with different conduction properties
- Separated by anatomy
- Functional unidirectional block and premature impulse
Two sets of tissue with different conduction properties
This could be scar tissue (e.g. from a ischemic insult, surgery etc) or normal but “different” tissue separated by normal anatomical boundaries – the classic examples being the slow pathway (SP) in AV Nodal re-entry and Cavo Tricuspid Isthmus (CIT) In right atrial flutter.
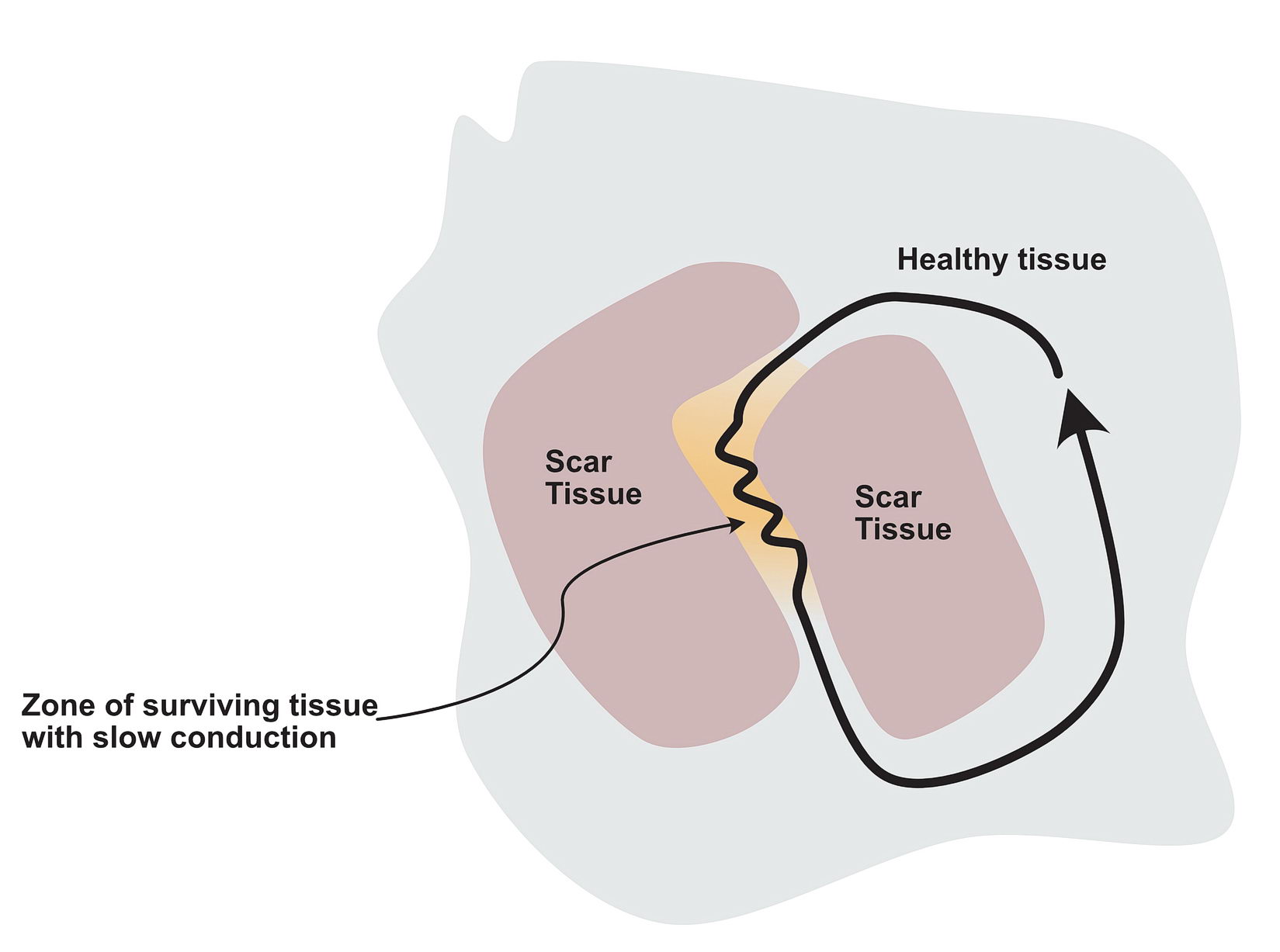
The critical distinction between the two groups of myocardium should be different conduction properties. Usually this means one group conducts slows and recovers fast whereas the other group conducts fast and recovers slowly. In a normal heart, majority of the tissues are fast conducting and slow recovering. That’s why when there is myocardial injury, slowly conducting tissues form this type of arrhythmia zones- i.e. the scar forms the obstruction (anatomical separation) and the still alive tissue in the scar forms slowly conducting segments. Collectively this facilitates re-entry – especially with premature impluses as described below.
Slowly conducting tissue recovers fast and fast conducting tissue takes time to recover
Anatomical separation of the tissue groups
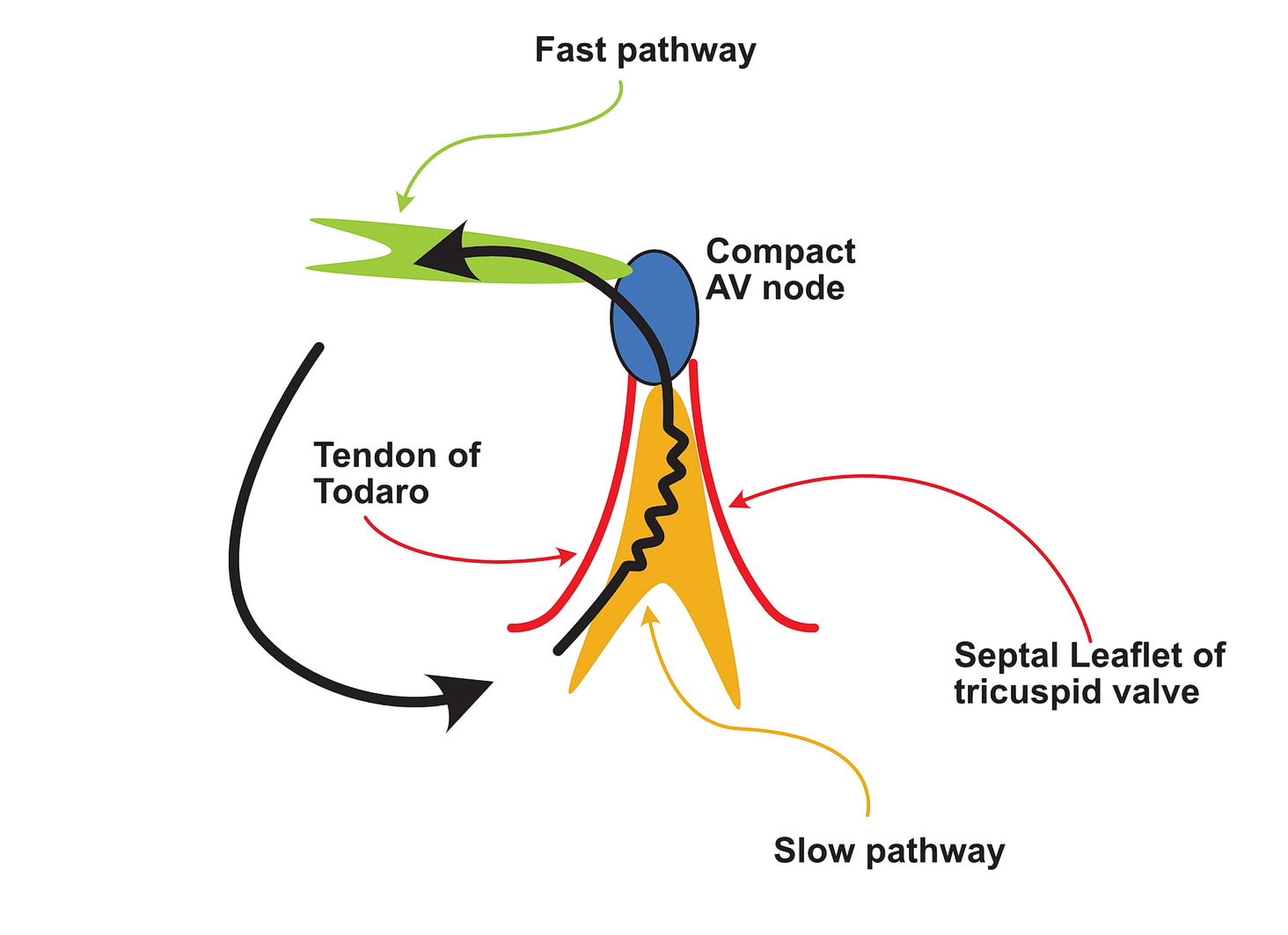
In a scar, a surviving but injured group of cells form the slow conducting tissue. Therefore the dead scar tissue forms the separation. However even in normal hearts, certain anatomical locations have differently conducting tissues which facilitate re-entry and they are separated by normal tissue with resistance to conduction. One good example is the slow pathway in AV nodal re-entry – where the slowly conducting tissue is bound by two barriers – the tendon of Todaro and the septal leaflet of the tricuspid valve (see figure). The other common place is the cavo-tricuspid-isthmus (CTI) where there is slow conduction and this facilitates re-entry. (see figure)

Functional Unidirectional Block and Premature Impulse
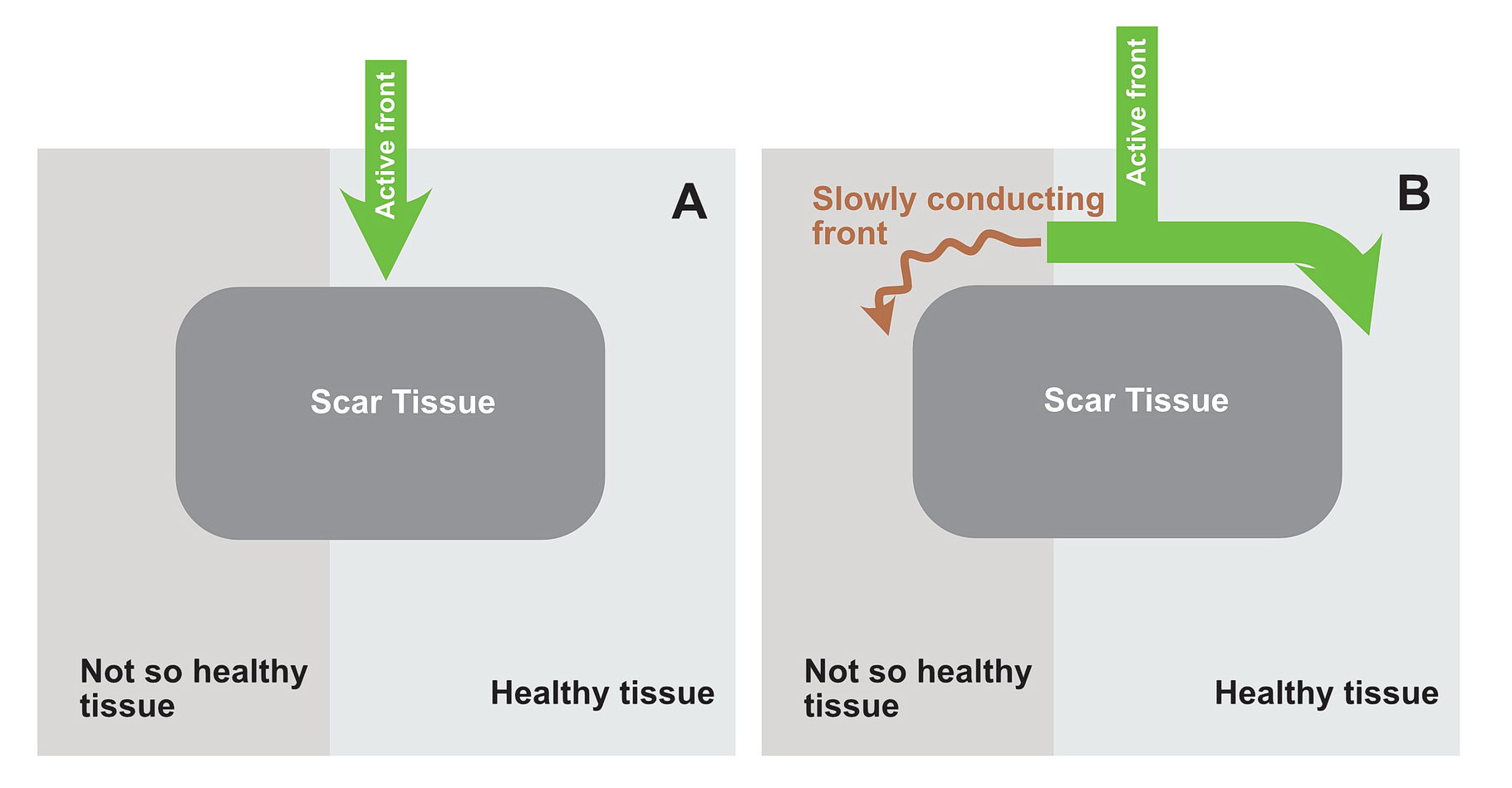
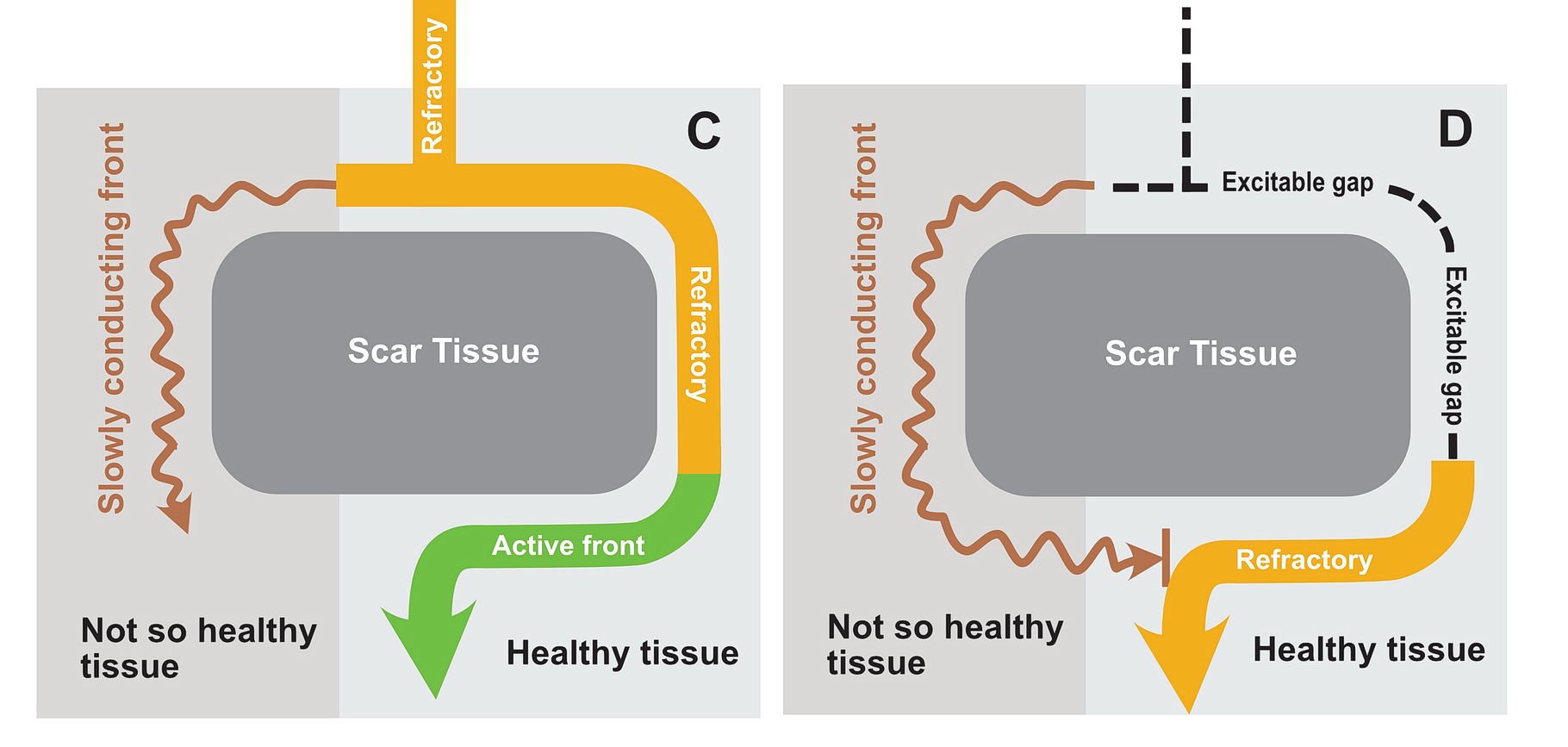
The premature impulse initiates the tachycardia loop by entering a functional unidirectional block. The following series of figures explain it.
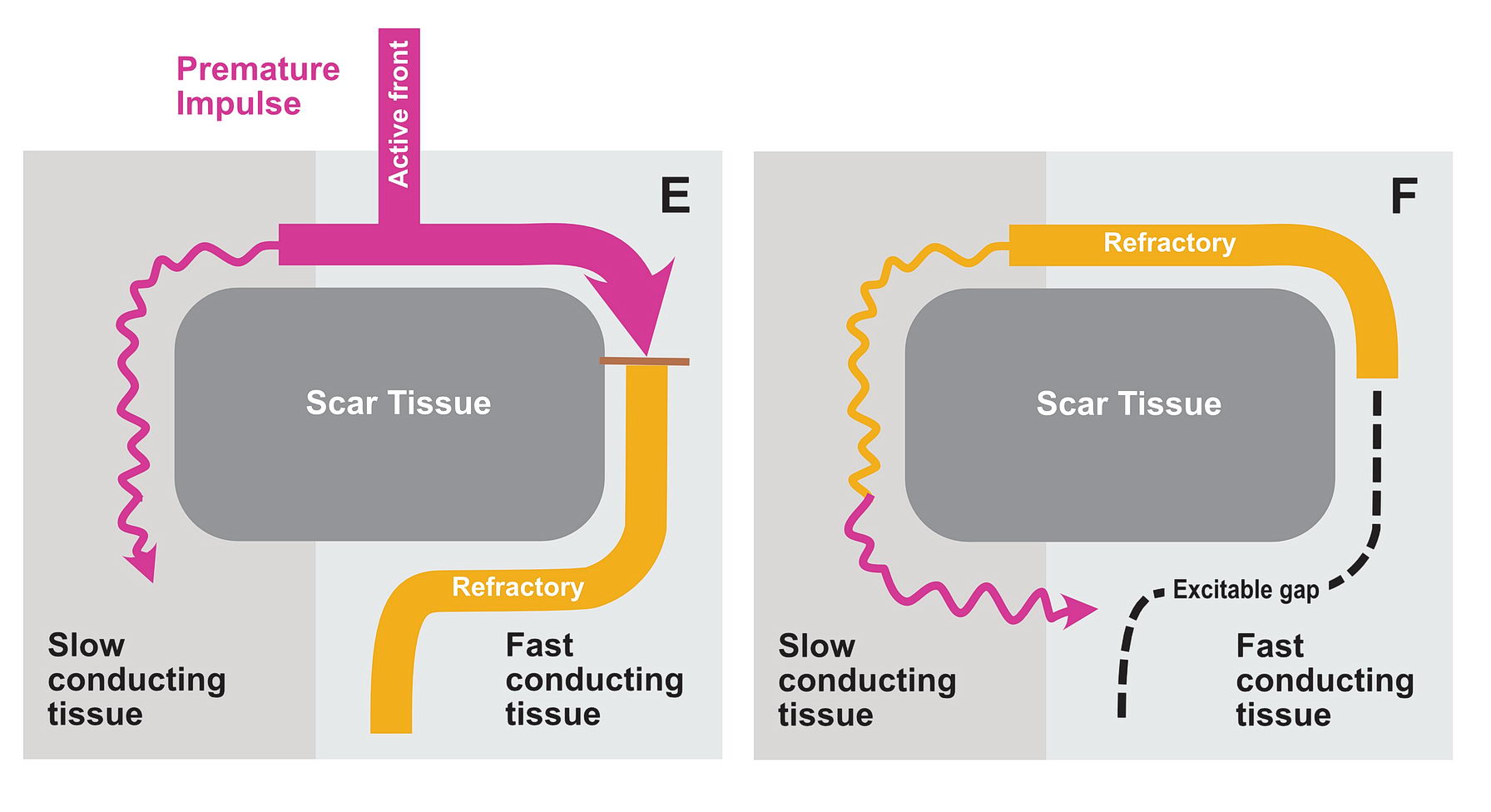
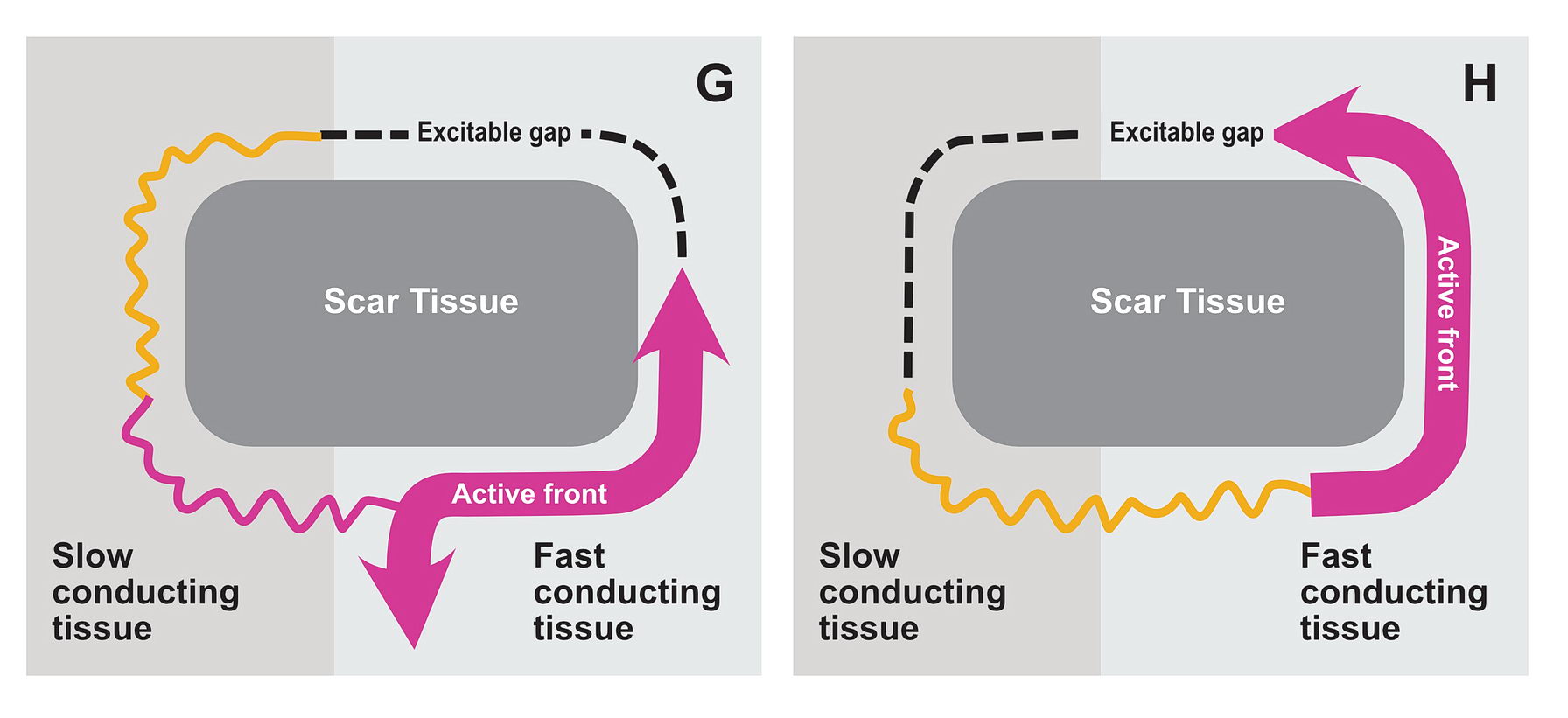
The functional unidirectional block is key for initiation of re-entry.
We routinely exploit this in the EP lab by putting premature complexes into the functional block to initiate tachycardia.
This also one mechanism how beta blockers reduce the incidence of arrhythmia – they prevent premature complexes which initiate re-entry
Although anatomically far apart, the same principle can be applied for accessory pathway mediated tachycardia (i.e. Atrioventricular Re-entrant tachycardia AVRT). One limb is the AV node and the other limb is the pathway. Normal sinus rhythm maintains a functional block in either limbs depending on the relative speed of conduction in the two limbs. Therefore a correctly timed PVC or PAC can initiate re-entry by entering the excitable gap during this functional block. In the following example, we assume the common situation of fast conducting, slowly recovering accessory pathway.
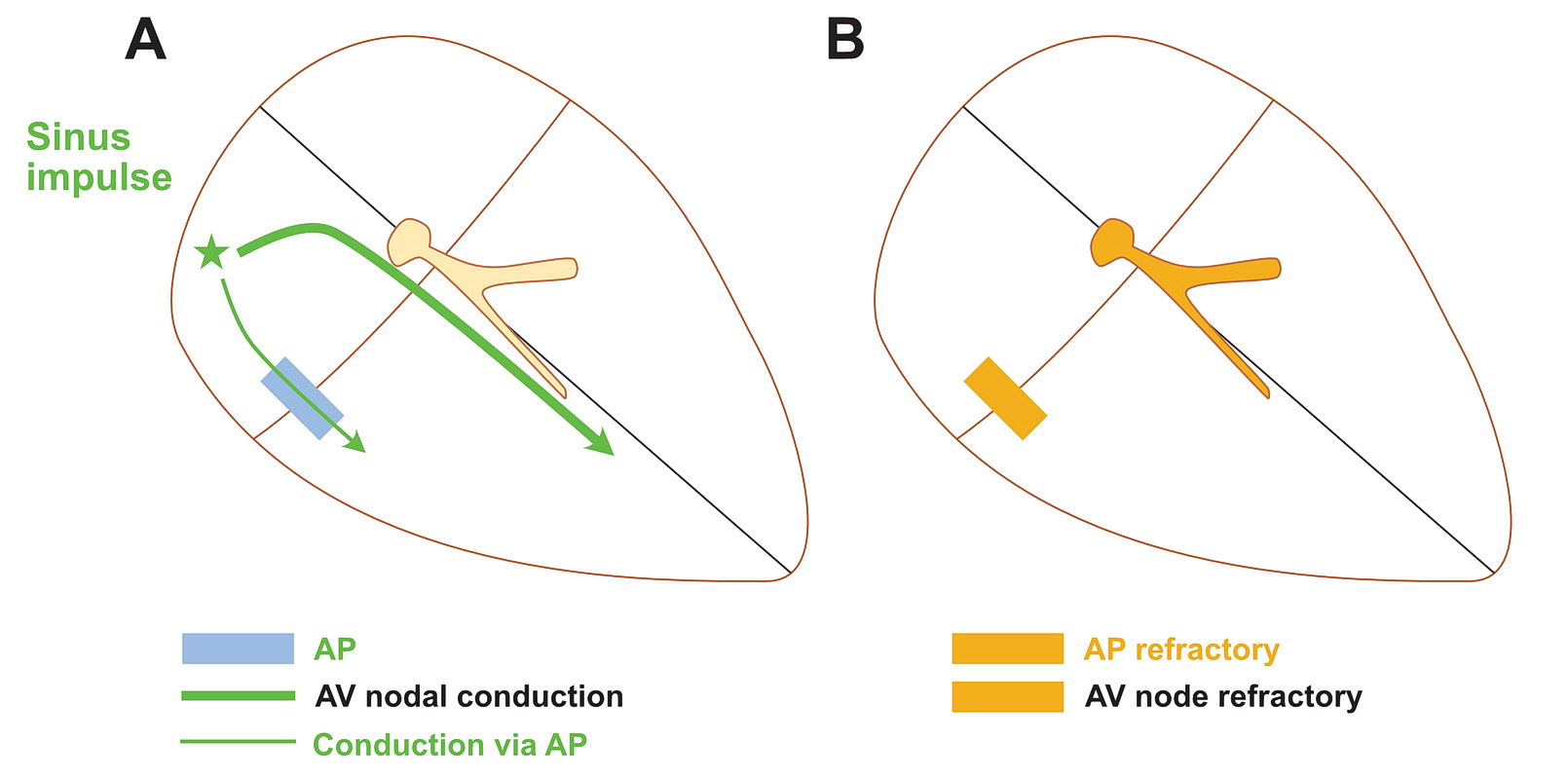
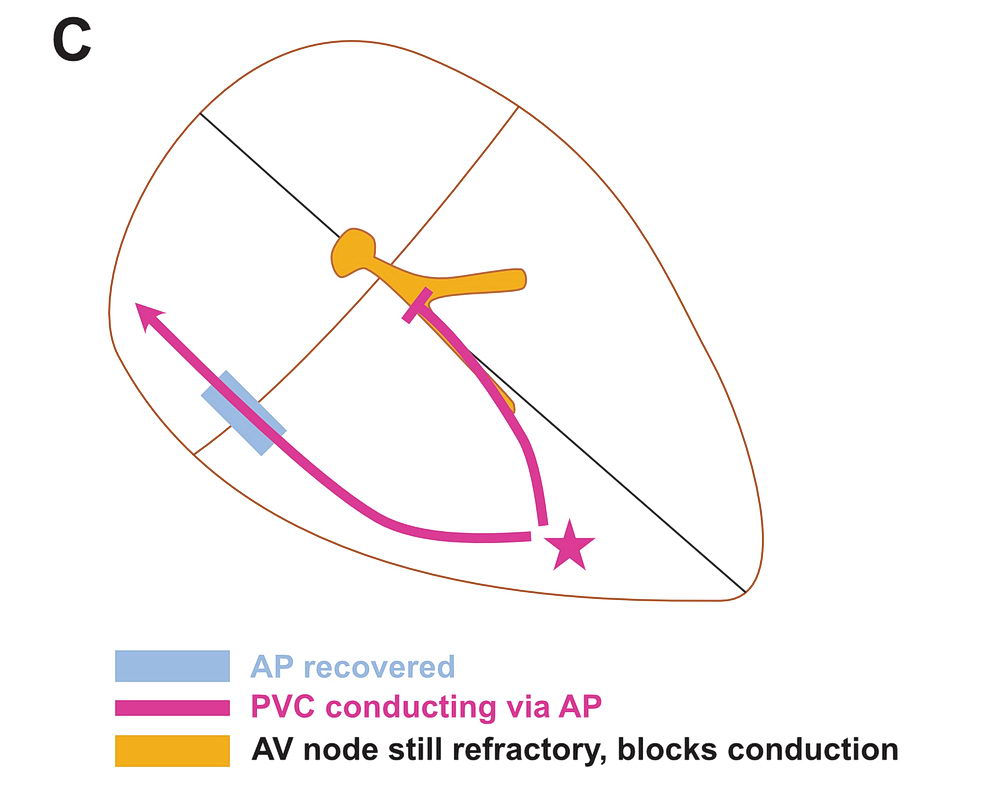
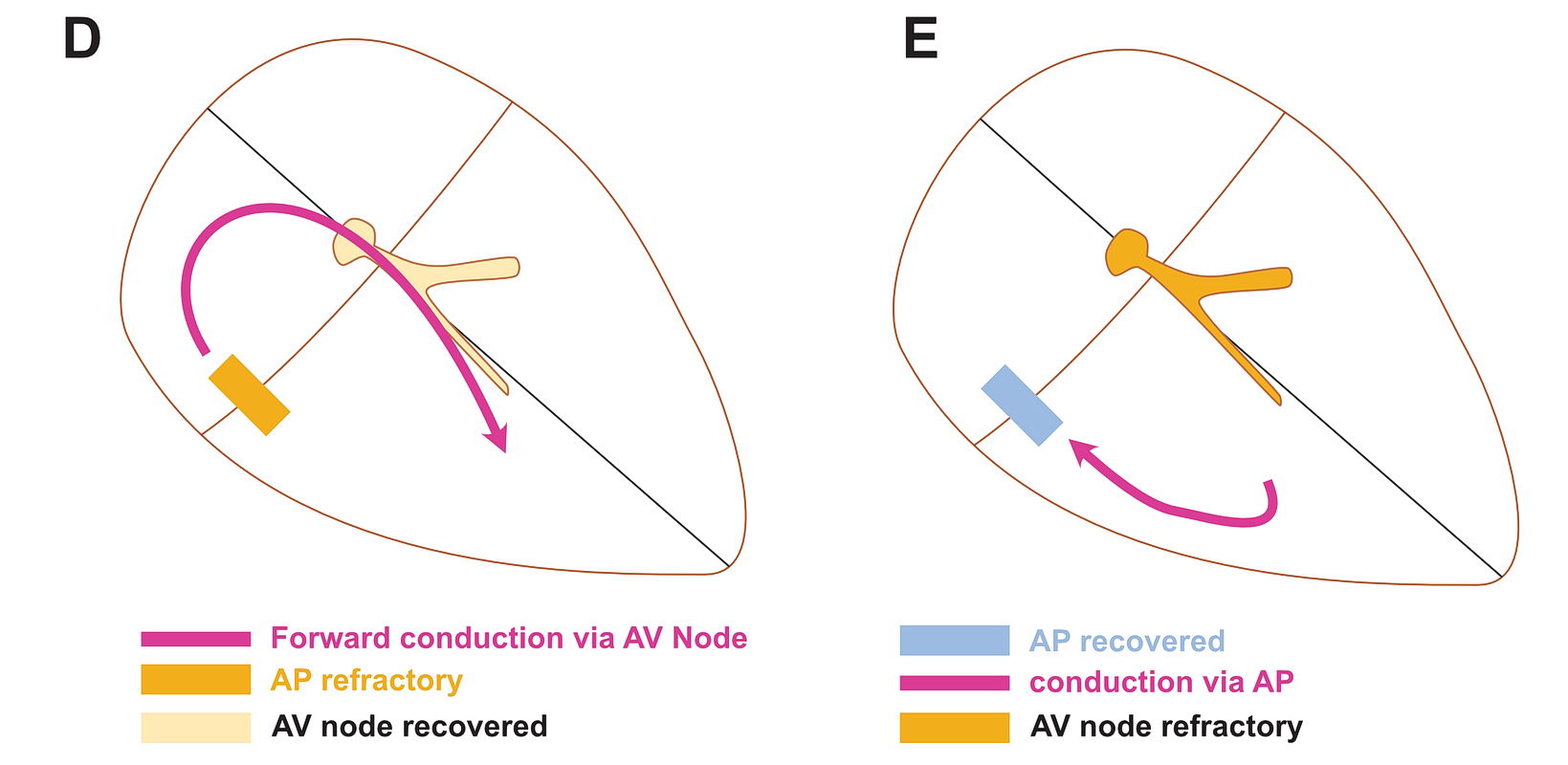
As shown above, PVCs can initiate tachycardia when the AV node is refractory. In addition, most APs conduct very happily from the ventricle to atrium – why? : Read the section on source-sink concept. Together taken (fast recovering limb and ease of conduction to V) is why extra-stimulus pacing in the ventricle easily induces tachycardia in patients with WPW
The same can happen with PACs in the atrium – but it is unusual as favored direction of travel across the AP would be V to A. If it did travel the other way, it would initiate an antidromic AVRT.
If the AP conduction is slower than the AV node, then PACs can initiate orthodromic AVRT – but again finding such AP would be rare – like in the case of PJRT.
To recap, re-entry substrate requires two myocardial tissue segments of different conducting properties connected at ends but separated by an obstruction. The common way of initiating re-entry is by premature complexes entering the circuit when one segment is functionally blocked.
The difference in conducting properties would be one tissue with fast conduction & delayed recovery and the other one with slow conduction & rapid recovery
How this applies to various tachycardia types will be discussed in each type of tachycardia.